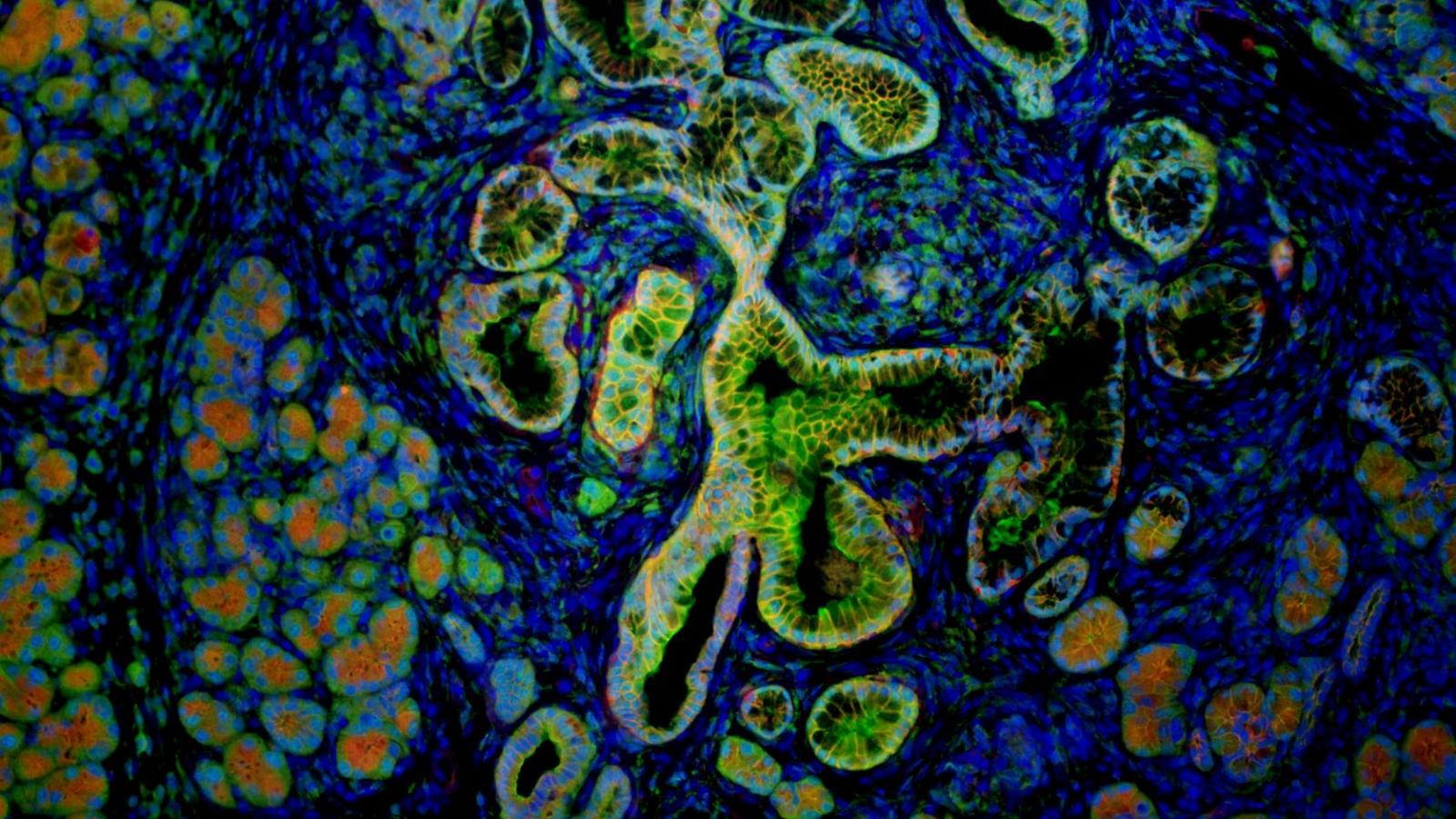
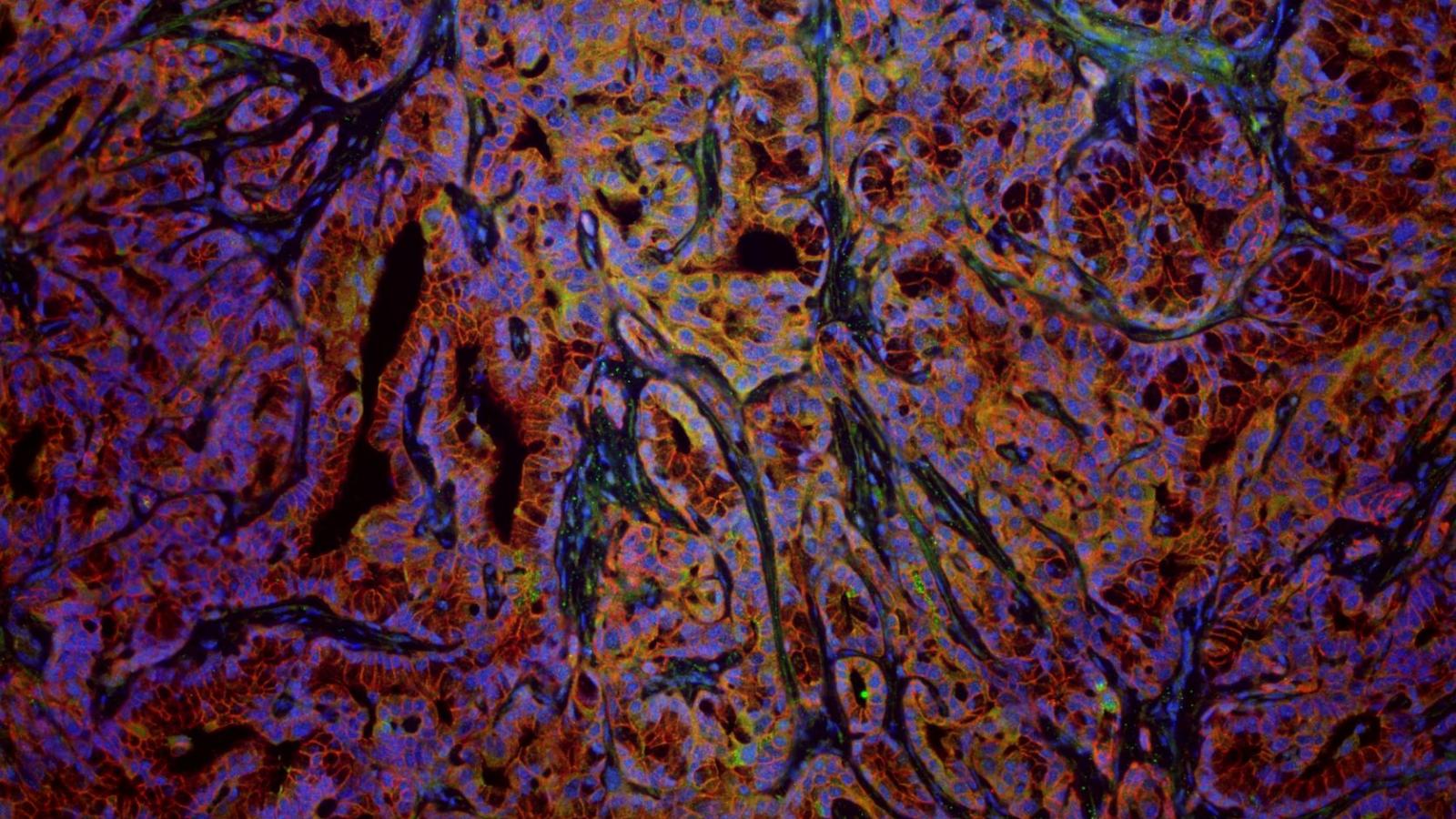
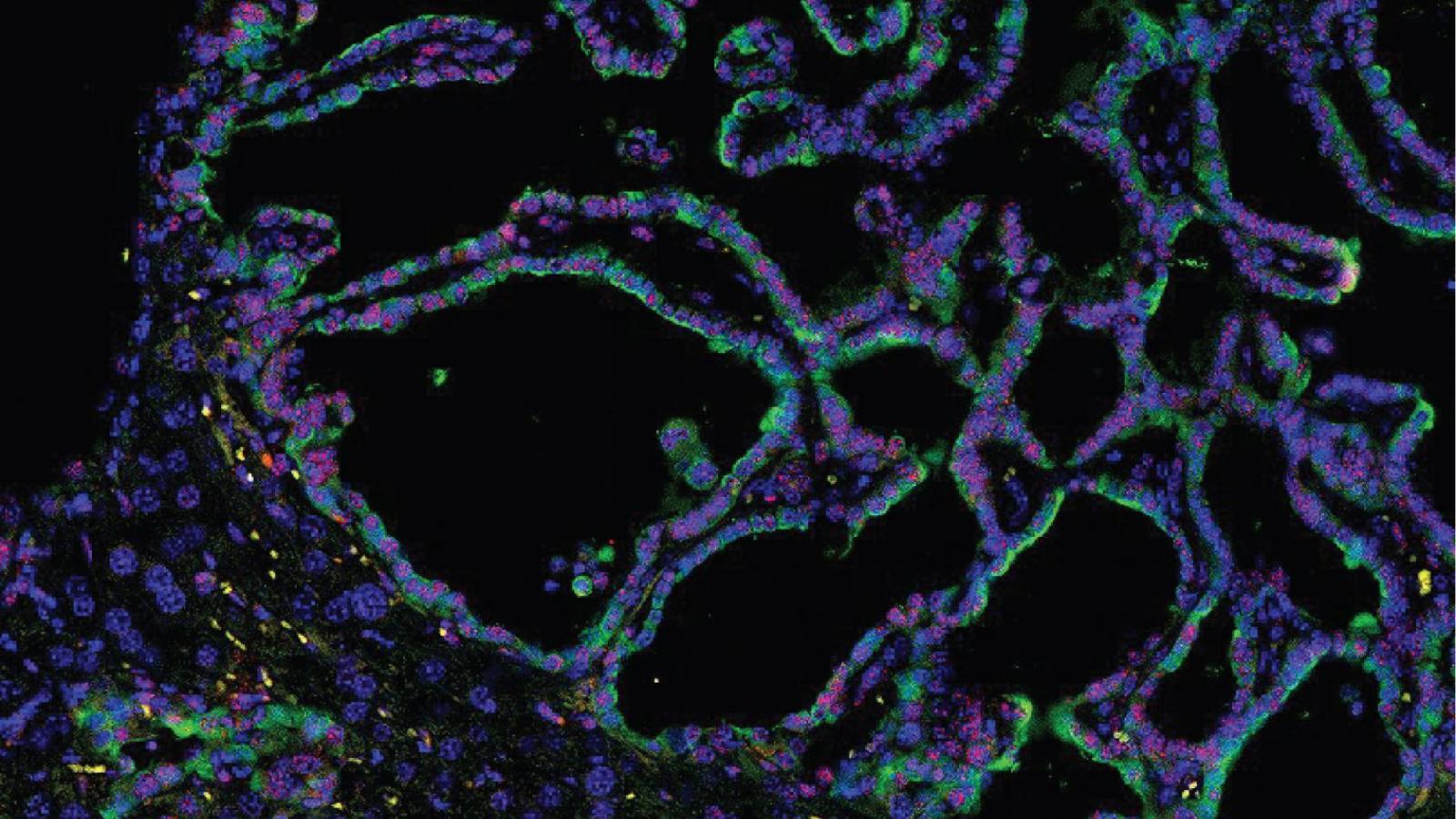
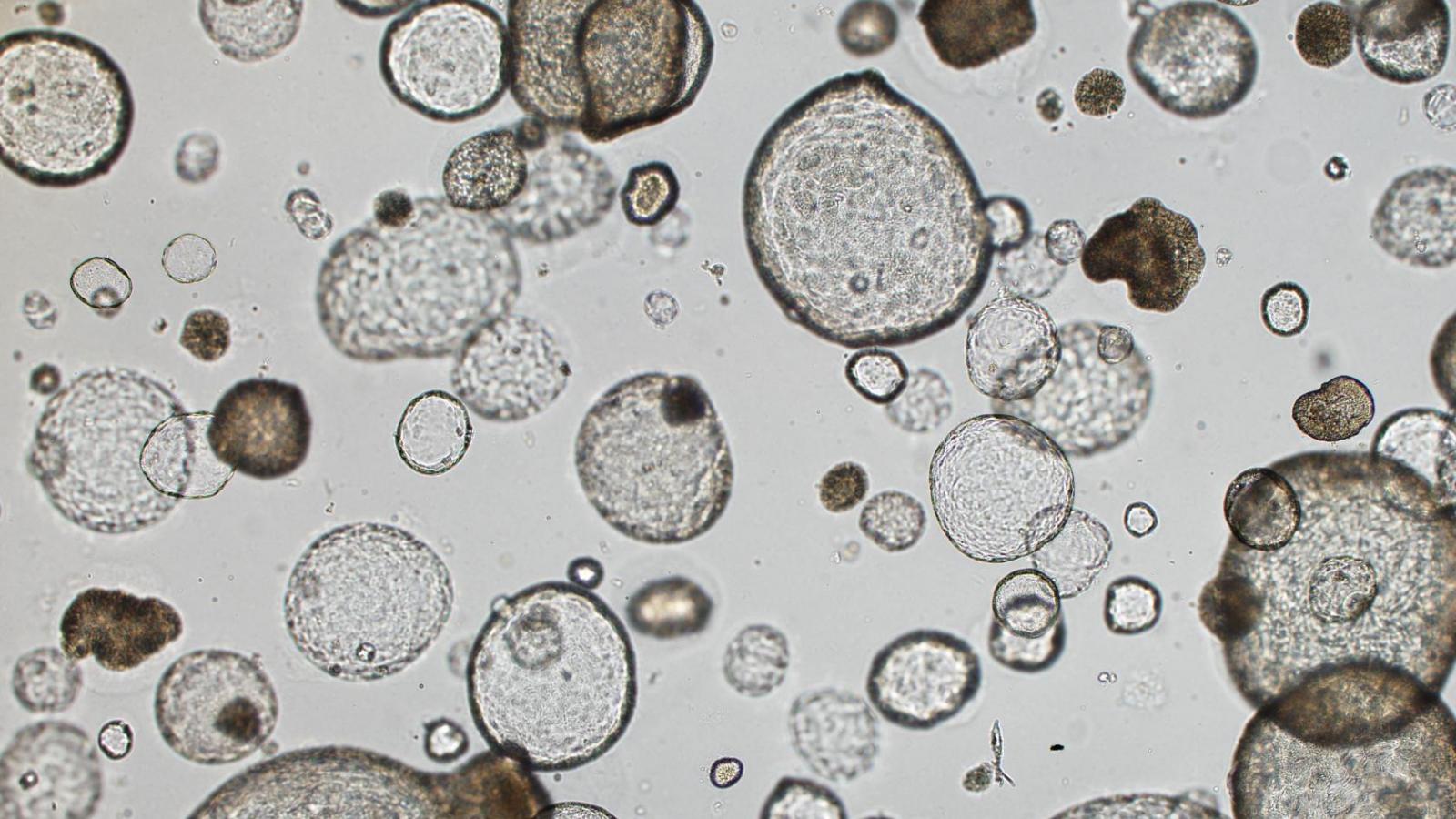
Rustgi Lab
The Rustgi laboratory has long-standing thematic interests in the cell-type and tissue-type specific actions of certain oncogenes (cyclin D1, EGFR) and tumor suppressor genes (p53, p120 catenin) in modulating the initiation, progression and metastasis of gastrointestinal cancers, especially upper GI, pancreatic and colon. We employ novel three-dimensional cell culture systems (mouse and human organoids and air-liquid interface) and genetically-engineered mouse models to investigate molecular mechanisms. These projects are translated into the objectives of improving molecular diagnostics and experimental therapeutics in patients.
Oncogenes and Tumor Suppressors in the Tumor Microenvironment
Tumor cell progression is illustrated by invasion into the extracellular matrix (ECM) or stoma. This then involves interrelated networks between tumor cells and diverse cell types in the tumor microenvironment. This cross-talk and cross-fertilization trigger a necessary cascade of events prior to dissemination of tumor cells into blood and lymphatic vessels, as well as local and distant metastasis. These include, but are not restricted to, immune cells/inflammatory cells, fibroblasts, endothelial cells, pericytes, neurons, and adipocytes. Our unified view is that p120ctn loss or mislocalization, either of which abrogates its tumor suppressor activities involved in the maintenance of the adherens junctions (complex with E-cadherin) fosters tumor initiation as revealed by our published work on the conditional loss of p120ctn in the mouse esophagus, resulting in invasive ESCC (with local metastasis to lymph nodes) accompanied by desmoplasia and the specific recruitment of myeloid derived suppressor cells (MDSCs) or immature myeloid cells.
Pancreatic Cancer & Cellular Plasticity
The exocrine pancreas has a remarkable ability to regenerate after injury, as illustrated in acute pancreatitis, and subsets of chronic pancreatitis. Acinar-ductal metaplasia (ADM) is critical in the ability of the exocrine pancreas to regenerate or permit progression to a preneoplastic state (pancreatic intraepithelial neoplasia or PanIN). In mouse models of pancreatic cancer, induction of either acute or chronic pancreatitis results in tissue-wide ADM that is followed by rapid repair (we designate this as “Adaptive” ADM). However, in the presence of oncogenic Kras, repair is impaired and ADM progresses to PanIN lesions (we designate this as “Oncogenic” ADM). Currently, the mechanisms underlying the formation of ADM and how ADM progresses to PanIN in the presence of mutant Kras remain unknown. Recently, our group performed gene expression analysis of murine ductal cells isolated from the developing pancreas, acute pancreatitis (ADM), and PanIN expressing oncogenic KrasG12D, and compared the expression profiles to that of normal pancreatic ductal cells, resulting in nearly 80 potential genes of interest. Prrx1 (paired-related homeobox 1) was the most differentially regulated transcription factor in all three processes, followed by Etv5, a member of the Ets family of transcriptional factors. Based upon compelling published and preliminary data, we hypothesize that Etv5 and Prrx1 are involved in the initiation and maintenance of ADM, respectively, following pancreatitis. Furthermore, we hypothesize that this regulation allows for subsequent transformation by oncogenic Kras, thereby promoting progression to PanIN.
RNA Binding Proteins in Colon Cancers
The intestinal epithelium is in a dynamic equilibrium of proliferation, differentiation and apoptosis along the crypt-villus gradient. Normal intestinal homeostasis is disturbed during states of infection, inflammation and malignant transformation (adenomatous polyps, colorectal cancer). The pathogenesis of sporadic colorectal cancer involves distinct pathways with characteristic genomic and genetic alterations. Despite significant advances in leveraging our understanding of these pathways for diagnostic, prognostic, and therapeutic strategies, colorectal cancer (CRC) remains a leading cause of cancer-related mortality. This underscores a specific need to identify and understand novel, therapeutically tractable pathways in intestinal homeostasis that may drive CRC. Our work has introduced and elucidated the novel role of mRNA binding proteins in intestinal/colonic epithelial homeostasis, as well as aberrations including hyperproliferation, altered metabolism and transformation. LIN28B, an mRNA binding protein, also critical in embryonic stem cells, post- transcriptionally regulates the let-7 microRNA family and results in suppression of differentiation. In turn, Let-7 microRNAs have diverse mRNA targets, including IMP1 (Igf2 mRNA binding protein-1), another mRNA binding protein. We have demonstrated that LIN28B and IMP1 separately drive tumor-initiating cell phenotypes associated with their roles in regulating proliferation and differentiation during normal homeostasis; however, it remains unclear if LIN28B-mediated hyperproliferation, altered differentiation, and associated tumorigenesis requires IMP1. In addition, it remains unknown if the tumor promoting or metastatic roles of IMP1 depend entirely on its regulation by Let-7 downstream of LIN28B. Our overarching hypothesis of this proposal is that LIN28B and IMP1 comprise cooperative roles in controlling post-transcriptionally the pathways (some known) associated with epithelial cell fate, which may favor malignant transformation.
Stem Cells in the Upper GI Tract
The esophageal lumen is lined by a stratified squamous epithelium comprised of proliferative basal cells that differentiate while migrating toward the luminal surface and eventually desquamate. Rapid epithelial renewal occurs, but the specific cell of origin that supports this high proliferative demand remains unknown. Herein, we have described a long-lived progenitor cell population in the mouse esophageal epithelium that is characterized by expression of keratin 15 (Krt15). Genetic in vivo lineage tracing revealed that the Krt15 promoter marks a long-lived basal cell population able to self-renew, proliferate, and generate differentiated cells, consistent with a progenitor/stem cell population. Transcriptional profiling demonstrated that Krt15+ basal cells are molecularly distinct from Krt15- basal cells. Depletion of Krt15-derived cells resulted in decreased proliferation, thereby leading to atrophy of the esophageal epithelium. Further, Krt15+ cells were radioresistant and contributed to esophageal epithelial regeneration following radiation-induced injury. These results establish the presence of a long-lived and indispensable Krt15+ progenitor cell population that provides additional perspective on esophageal epithelial biology and the widely prevalent diseases that afflict this epithelium.
Tumor Metastasis and Metastatic Organotropism
Metastatic organotropism, a phenomenon defined by the intrinsic propensity of tumor cells to selectively metastasize to different organs, is poorly understood despite being first described in Stephen Paget’s famous 1889 “seed and soil” hypothesis. Using novel mouse models of pancreatic cancer, we have demonstrated that liver and lung metastatic organotropism is dependent on the E-CADHERIN regulating protein p120 catenin (p120ctn). Complete loss of p120ctn destabilizes membranous E-CAD leading to a preference towards lung metastases. In contrast, heterozygous deletion of p120ctn, a state sufficient for E-CAD-mediated cellular adhesion, allows for both lung and liver metastases. Collectively, these results suggest that cells undergo epithelial-to-mesenchymal transition (EMT) and are thus in a mesenchymal state favor lung metastastasis, while those that are able to undergo the reverse mesenchymal-to-epithelial transition (MET) and thus return to an epithelial state can metastasize to either liver or lung. We are currently undergoing various screens to determine the downstream mechanisms that govern both EMT-MET axis as well as metastatic organotropism.
Selected Publications
The LIN28B-IMP1 Post-Transcriptional Regulon Has Opposing Effects on Oncogenic Signaling in the Intestine
RNA-binding proteins (RBPs) are expressed broadly during both development and malignant transformation, yet their mechanistic roles in epithelial homeostasis or as drivers of tumor initiation and progression are incompletely understood. Here we describe a novel interplay between RBPs LIN28B and IMP1 in intestinal epithelial cells. Ribosome profiling and RNA sequencing identified IMP1 as a principle node for gene expression regulation downstream from LIN28B. In vitro and in vivo data demonstrate that epithelial IMP1 loss increases expression of WNT target genes and enhances LIN28B-mediated intestinal tumorigenesis, which was reversed when we overexpressed IMP1 independently in vivo. Furthermore, IMP1 loss in wild-type or LIN28B-overexpressing mice enhances the regenerative response to irradiation. Together, our data provide new evidence for the opposing effects of the LIN28B–IMP1 axis on post-transcriptional regulation of canonical WNT signaling, with implications in intestinal homeostasis, regeneration and tumorigenesis.
IL-6 Mediates Cross-Talk Between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment
The tumor microenvironment (TME) plays a major role in the pathogenesis of multiple cancer types, including upper-gastrointestinal (GI) cancers that currently lack effective therapeutic options. Cancer-associated fibroblasts (CAF) are an essential component of the TME, contributing to tumorigenesis by secreting growth factors, modifying the extracellular matrix, supporting angiogenesis, and suppressing anti-tumor immune responses. Through an unbiased approach, we have established that IL-6 mediates crosstalk between tumor cells and CAF not only by supporting tumor cell growth, but also by promoting fibroblast activation. As a result, IL-6 receptor (IL-6Rα) and downstream effectors offer opportunities for targeted therapy in upper-GI cancers. IL-6 loss suppressed tumorigenesis in physiologically relevant 3D organotypic and 3D tumoroid models and murine models of esophageal cancer. Tocilizumab, an anti-IL-6Rα antibody, suppressed tumor growth in vivo in part via inhibition of STAT3 and MEK/ERK signaling. Analysis of a pan-cancer TCGA dataset revealed an inverse correlation between IL-6 and IL-6Rα overexpression and patient survival. Therefore, we expanded evaluation of tocilizumab to head-and-neck squamous cell carcinoma patient-derived xenografts and gastric adenocarcinoma xenografts, demonstrating suppression of tumor growth and altered STAT3 and ERK1/2 gene signatures. We used small molecule inhibitors of STAT3 and MEK1/2 signaling to suppress tumorigenesis in the 3D organotypic model of esophageal cancer. We demonstrate that IL-6 is a major contributor to the dynamic crosstalk between tumor cells and CAF in the TME. Our findings provide a translational rationale for inhibition of IL-6Rα and downstream signaling pathways as a novel targeted therapy in oral-upper-GI cancers.
Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer
The regulation of metastatic organotropism in pancreatic ductal a denocarcinoma (PDAC) remains poorly understood. We demonstrate, using multiple mouse models, that liver and lung metastatic organotropism is dependent upon p120catenin(p120ctn)-mediated epithelial identity. Mono-allelic p120ctn loss accelerates KrasG12D-driven pancreatic cancer formation and liver metastasis. Importantly, one p120ctn allele is sufficient for E-CADHERIN-mediated cell adhesion. By contrast, cells with bi-allelic p120ctn loss demonstrate marked lung organotropism; however, rescue with p120ctn isoform 1A restores liver metastasis. In a p120ctn-independent PDAC model, mosaic loss of E-CADHERIN expression reveals selective pressure for E-CADHERIN-positive liver metastasis and E-CADHERIN-negative lung metastasis. Furthermore, human PDAC and liver metastases support the premise that liver metastases exhibit predominantly epithelial characteristics. RNA-seq demonstrates differential induction of pathways associated with metastasis and epithelial-to-mesenchymal transition in p120ctn-deficient versus p120ctn-wild-type cells. Taken together, P120CTN and E-CADHERIN mediated epithelial plasticity is an addition to the conceptual framework underlying metastatic organotropism in pancreatic cancer.
Mouse Intestinal Krt15+ Crypt Cells Are Radio-Resistant and Tumor Initiating
Two principal stem cell pools orchestrate the rapid cell turnover in the intestinal epithelium. Rapidly cycling Lgr5+ stem cells are intercalated between the Paneth cells at the crypt base (CBCs) and injury-resistant reserve stem cells reside above the crypt base. The intermediate filament Keratin 15 (Krt15) marks either stem cells or long-lived progenitor cells that contribute to tissue repair in the hair follicle or the esophageal epithelium. Herein, we demonstrate that Krt15 labels long-lived and multipotent cells in the small intestinal crypt by lineage tracing. Krt15+ crypt cells display self-renewal potential in vivo and in 3D organoid cultures. Krt15+ crypt cells are resistant to high-dose radiation and contribute to epithelial regeneration following injury. Notably, loss of the tumor suppressor Apc in Krt15+ cells leads to adenoma and adenocarcinoma formation. These results indicate that Krt15 marks long-lived, multipotent, and injury-resistant crypt cells that may function as a cell of origin in intestinal cancer.
CD38+ M-MDSC Expansion Characterizes a Subset of Advanced Colorectal Cancer Patients
Myeloid-derived suppressor cells (MDSCs) are a population of immature immune cells with several protumorigenic functions. CD38 is a transmembrane receptor–ectoenzyme expressed by MDSCs in murine models of esophageal cancer. We hypothesized that CD38 could be expressed on MDSCs in human colorectal cancer (CRC), which might allow for a new perspective on therapeutic targeting of human MDSCs with anti-CD38 monoclonal antibodies in this cancer. METHODS. Blood samples were collected from 41 CRC patients and 8 healthy donors, followed by peripheral blood mononuclear cell (PBMC) separation. Polymorphonuclear (PMN-) and monocytic (M-) MDSCs and CD38 expression levels were quantified by flow cytometry. The immunosuppressive capacity of M-MDSCs from 10 CRC patients was validated in a mixed lymphocyte reaction (MLR) assay. RESULTS. A significant expansion of CD38+ M-MDSCs and a trend of expansion of CD38+ PMNMDSCs (accompanied by a trend of increased CD38 expression on both M- and PMN-MDSCs) were observed in PBMCs of CRC patients when compared with healthy donors. The CD38+ M-MDSCs from CRC patients were found to be immunosuppressive when compared with mature monocytes. CD38+ M- and PMN-MDSC frequencies were significantly higher in CRC patients who previously received treatment when compared with treatment-naive patients. CONCLUSIONS. This study provides a rationale for an attempt to target M-MDSCs with an anti-CD38 monoclonal antibody in metastatic CRC patients.
Long-Lived Keratin 15+ Esophageal Progenitor Cells Contribute to Homeostasis and Regeneration
The esophageal lumen is lined by a stratified squamous epithelium comprised of proliferative basal cells that differentiate while migrating toward the luminal surface and eventually desquamate. Rapid epithelial renewal occurs, but the specific cell of origin that supports this high proliferative demand remains unknown. Herein, we have described a long-lived progenitor cell population in the mouse esophageal epithelium that is characterized by expression of keratin 15 (Krt15). Genetic in vivo lineage tracing revealed that the Krt15 promoter marks a long-lived basal cell population able to self-renew, proliferate, and generate differentiated cells, consistent with a progenitor/stem cell population. Transcriptional profiling demonstrated that Krt15+ basal cells are molecularly distinct from Krt15- basal cells. Depletion of Krt15-derived cells resulted in decreased proliferation, thereby leading to atrophy of the esophageal epithelium. Further, Krt15+ cells were radioresistant and contributed to esophageal epithelial regeneration following radiation-induced injury. These results establish the presence of a long-lived and indispensable Krt15+ progenitor cell population that provides additional perspective on esophageal epithelial biology and the widely prevalent diseases that afflict this epithelium.
Lab Members
Current Lab Members
Michelle Cohen
- PhD Student
Katherine Cunningham
- MD/PhD Student
Karen Dunbar, PhD
- Associate Research Scientist
Gizem Efe
- PhD Student
Emily Esquea, PhD
- Postdoctoral Research Scientist
Raul Navridas Fernandez de Bobadil, PhD
- Postdoctoral Research Scientist
Constanza Nicole Tapia Contreras, PhD
- Postdoctoral Research Scientist
Noriyuki Nishwaki, MD
- Postdoctoral Research Scientist
Ben Rhoades, MB
- Lab Manager
Alice Shin, PhD
- Postdoctoral Research Scientist