Using Patient-Derived Organoids, Oncologists Can Predict a Tumor’s Response to Treatment
Organoids Offer a Patient-Specific Roadmap to Cancer Treatment Decisions
Researchers at the Herbert Irving Comprehensive Cancer Center (HICCC) at NewYork-Presbyterian/Columbia University Irving Medical Center are pioneering new tools for treating head and neck cancers.
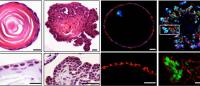
Using 3-dimensional representations of a patient’s tumor, known as organoids, researchers can mimic a tumor’s biological activity in vitro. By evaluating an organoid’s response to various drugs, physicians are better equipped to predict a tumor’s response to treatment options.
“Patient-derived organoids have profound potential in personalized medicine,” says Anuraag Suhrid Parikh, MD, assistant professor of otolaryngology-head and neck surgery at Columbia University Irving Medical Center (CUIMC). “In head and neck cancer, utilizing patient-derived organoids to help predict response to non-surgical therapies may help with decision-making in cancer types such as HPV-positive oropharynx cancer or early stage larynx cancer, in which both surgical and non-surgical therapies are options.”
How organoids are used to guide treatment decisions
Clinicians obtain tissue from a patient at the same time a biopsy is taken for pathologic diagnosis.
“Once we have a tissue specimen from a patient,” says Hiroshi Nakagawa, MD, PhD, associate professor of medicine at Columbia's Vagelos College of Physicians and Surgeons and a member of the HICCC, “we can rapidly grow a patient’s original tumor into a 3D structure in test tubes. This structure, or organoid, mimics the original tumor, and we can then offer analyses, molecular-level sequencing, or conduct treatment experiments by using these organoids as our disease models.”
The entire process takes 14 days to complete—the timeframe of a standard patient’s workup. The HICCC derives organoids with an 80% success rate, far exceeding the success rate of other programs in the nation.
Organoids help predict tumor response
“One key aspect of this is our ability to test, identify, and potentially predict the drugs that will work best for each cancer patient,” Dr. Nakagawa says. In the Nakagawa lab, researchers Samuel Flashner, PhD, and Cecilia Martin, MS, are leading efforts to generate a well-characterized library of head and neck and other cancer patient-derived organoids and optimize experimental conditions to test multiple therapeutics utilizing the organoids.
For specimens containing immune cells, it may be possible to model the effects of immune checkpoint inhibitors on tumor growth.
"If we are able to optimize an early passage organoid platform that retains some immune cells, another goal would be to reliably model the impact of immune checkpoint inhibitors, as 15% of recurrent/metastatic patients have a durable response—but we still don’t have reliable biomarkers to predict who those 15% will be," says Dr. Parikh.
Dr. Nakagawa’s research focuses also on the early detection of cancer.
“We want to know what initiates early-stage cancer development and how best to detect those invisible lesions,” he says. “Pathology may not always be able to see some of these changes at the very early stage in cancer development. With technology advancements, now we are hoping to prove that patient-derived organoids can reveal these changes.”
Aspiring to the ultimate goal of cancer care, Dr. Nakagawa concludes, “With this knowledge, we can get closer to a cure or a way to prevent cancer progression."



